
Hydrated copper sulphate crystals Stock Image C010/9660 Science Photo Library
It is often used to grow crystals in schools and in copper plating experiments despite its toxicity. Copper sulfate is often used to demonstrate an exothermic reaction, in which steel wool or magnesium ribbon is placed in an aqueous solution of CuSO4. It is used to demonstrate the principle of mineral hydration.

The Best Way to Grow Big Copper Sulfate Crystals Crystalverse
Preparation 1: copper (II) sulfate. Equipment required for neutralising copper (II) oxide and magnesium carbonate. Add 20 cm 3 of 0.5 M sulfuric acid to the 100 cm 3 beaker and heat carefully on the tripod with a gentle blue flame until nearly boiling. Be very careful not to knock the tripod while the beaker is supported by it.

Copper Sulfate Crystal, Copper Sulfate, blue vitriol, कॉपर सल्फेट SHREEJI INDUSTRIES, Rajkot
Copper sulfate is a chemical compound that produces stunning blue crystals. Plus, it's really easy to grow them. In fact, it's one of the easiest compounds to grow crystals with. There are already many guides on growing copper sulfate online. But most of them do not have detailed procedures. Plus, the crystals that they show are usually imperfect.

Macrophoto Of Copper Sulphate Crystals Photograph by Martin Dohrn/science Photo Library Pixels
Copper sulfate should be used as a pond algae treatment on actively growing algae in water temperature above 60F. When getting rid of algae in a pond, it is always important to treat 1/2 of the pond algae at a time to avoid killing too much algae and depleting the water of oxygen. Granular copper can be applied in one of three different ways.

Copper Sulphate Crystal, 50 Kg, Grade Standard Industrial Grade, Rs 145 /kg ID 4754570762
Copper (II) sulfate. 650°C decomp. Copper (II) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Its chemical formula is CuSO 4. It contains copper in its +2 oxidation state. It also contains sulfate ions. It is a blue solid that can kill fungi.

Copper sulfate fasgrade
This video introduces the concepts of supersaturation, nucleation and crystallisation. The amount of solute that can dissolve in a liquid, i.e. its solubilit.

Copper sulphate crystals Stock Image C036/3512 Science Photo Library
What to do? There are two scenarios possible. In the first one, crystals form on the bottom of the test tube instead of growing on the wire. If so, carefully cut the wire to expose a clean copper surface from the end you stick into the test tube. Repeat the experiment starting from the step 3 of the instruction.

The Extended Guide to Growing Copper Sulfate Crystals Crystalverse
In its solid, crystal-shaped stone form (known as a pentahydrate), copper sulfate is known as blue stone or blue vitriol for its blue color. In this form, it's a popular raw material for producing other types of copper salts. Copper sulfate uses include stopping the growth of fungus and bacteria.

How to grow a large crystal of copper (II) sulphate in 5 days
A similar experiment is included in the MEL Chemistry subscription.For safe experiments to do at home sign up to MEL Science here: https://goo.gl/pxsxaeWe al.

The Best Way to Grow Big Copper Sulfate Crystals Crystalverse
Procedure for beautiful copper crystals. Prepare a solution of copper sulfate pentahydrate. You won't need much. I usually dissolve 7.5 grams of copper sulfate in 50ml of distilled water. Pour a small amount of copper sulfate solution into the petri dish, soaking the filter paper. After a few seconds, pour out the solution, so you are left.

Hydrated Copper Sulphate Crystals Photograph by Dirk Wiersma
WE have been able to determine the structure of copper sulphate pentahydrate, which was the first crystal used by Friedrich and Knipping to diffract X-rays. The unit cell has dimensions: α0 = 6.

Hydrated copper (II) sulphate crystals Stock Image C004/7690 Science Photo Library
Edexcel Salts - Edexcel Core practical - making copper sulfate crystals Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants.

Core practical making copper sulfate crystals Salts Edexcel GCSE Combined Science
A solution of copper sulphate drains away from such heaps. Commercially copper sulphate contains 25 % metallic copper and is sold with a guaranteed minimum purity of 98 % copper sulphate. It is produced in a number of grades varying from large crystal lumps, of 25 mm or more in diameter from which it appropriately derives the name bluestone, to.

After a year of waiting, I got this beautiful three inch copper sulfate crystal. chemistry
Making copper sulfate crystals There are a number of ways that you could make copper sulfate crystals in Chemistry. This is an outline of the required steps to undertake one of these methods.
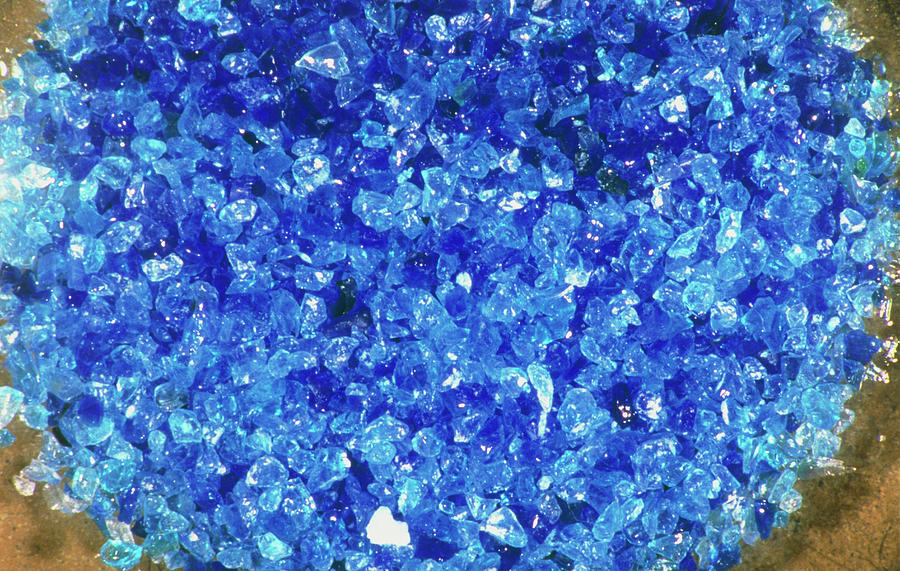
Hydrated Copper Sulphate Crystals Photograph by David Leah/science Photo Library. Pixels
Method. First identify your solute and solvent. Solute - copper sulfate. Solvent - water. Make a saturated solution by adding and stirring copper sulfate into very hot water until no more will dissolve. Pour the solution into a jar containing a piece of string hanging out over the edge and wait a few days for crystals to grow.
/copper-sulphate-crystals-926964832-5c09287846e0fb000141eb9a.jpg)
How to Grow Blue Copper Sulfate Crystals
Growing copper crystals 2,3. This is a simple, but colourful, redox reaction (see front cover). Copper(II) sulfate reacts with iron. Cotton is used to separate the reactants with a nifty layering effect in the final product. Students will be able to watch the products form over a couple of days.