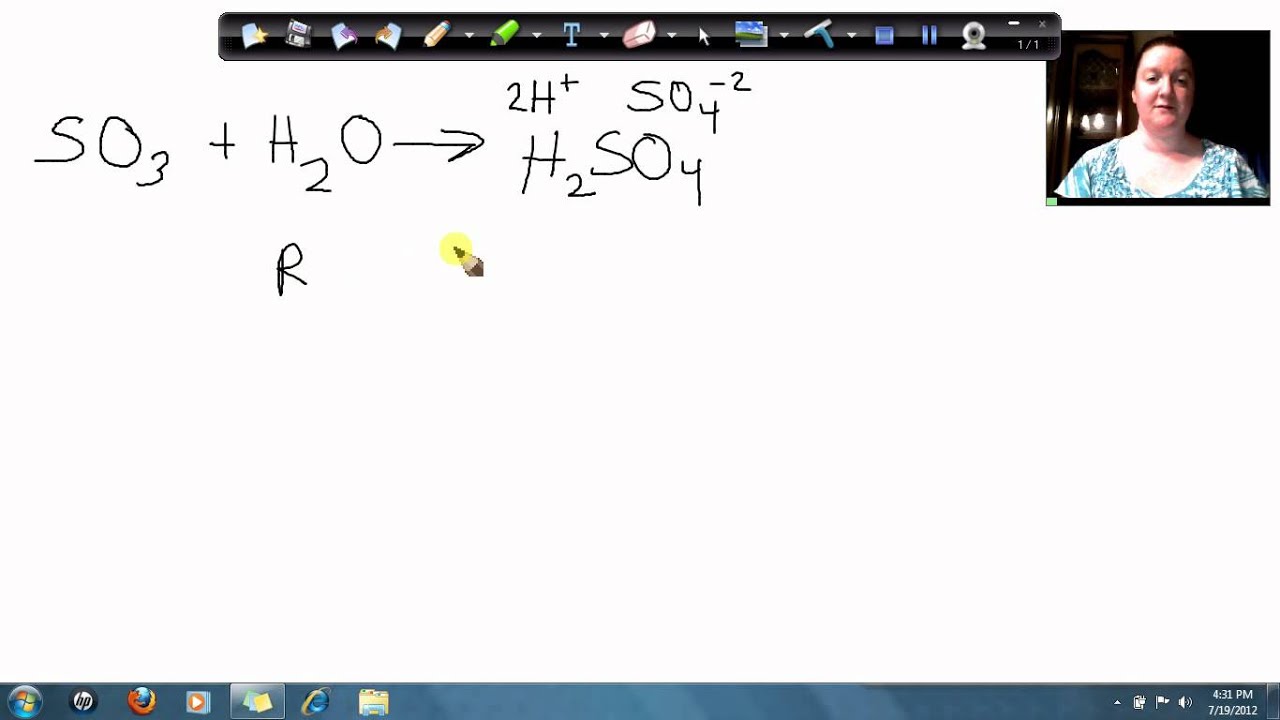
maxresdefault.jpg
The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). [7] When the formal charge is non-zero, the S-O bonding is assumed to be delocalized.

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur
Description Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. It reacts violently with water to form sulfuric acid with the release of heat. It is corrosive to metals and tissue. It causes eye and skin burns.
How to calculate the molar mass of sulfur trioxide YouTube
Formula: O 3 S Molecular weight: 80.063 IUPAC Standard InChI:InChI=1S/O3S/c1-4 (2)3 IUPAC Standard InChIKey:AKEJUJNQAAGONA-UHFFFAOYSA-N CAS Registry Number: 7446-11-9 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java Javascript . Other names: Sulfur trioxide
Sulfur trioxide molecule Stock Vector Images Alamy
SO3 + H2O → H2SO4 Sulphur trioxide reacts with a base of sodium hydroxide and forms sodium hydrogen phosphate. The chemical equation is given below. SO3 + NaOH → NaHSO4 Uses of Sulphur Trioxide - SO 3 Used as a bleaching agent to remove residual hydrogen peroxide, or as a digesting agent to separate pulp from lignin.

Sulfur Trioxide So3 Gas Moleculestick Model Stock Vector (Royalty Free
Word Equation. Sulfur + Dioxygen = Sulfur Trioxide. S + O2 = SO3 is a Synthesis reaction where two moles of Sulfur [S] and three moles of Dioxygen [O 2] combine to form two moles of Sulfur Trioxide [SO 3] Show Chemical Structure Image. Reaction Type. Synthesis. Redox (Oxidation-Reduction) Reaction.

How to Write the Formula for Sulfur Trioxide YouTube
Example 7.3.1 7.3. 1. Transfer the following symbolic equations into word equations or word equations into symbolic equations. HCl(aq) + NaOH(aq) → NaCl(aq) +H2O(l) HCl ( a q) + NaOH ( a q) → NaCl ( a q) + H 2 O ( l) Gaseous propane, C3H8 C 3 H 8, burns in oxygen gas to produce gaseous carbon dioxide and liquid water.

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
Other names: Sulfur trioxide Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page:. By formula: (CAS Reg. No. 14996-02-2 • 4294967295 O 3 S) + O 3 S = CAS Reg. No. 14996-02-2. Quantity Value Units Method Reference
Sulfur Trioxide Molecular Geometry Molecule Sulfur Dioxide, PNG
Figure 9.8.1 9.8. 1: The structure of sulfur dioxide. The modest boiling temperature of SO 2 (-10 °C) means that it is readily liquefied and easily kept as a liquid at room temperature under a slight pressure. The liquid is associated by dipole-dipole attractions due to the polar nature of SO 2. Liquid SO 2 is a good solvent due to the.

SOLVEDWrite a balanced equation for the reaction of sulfur trioxide
Formula: O 3 S Molecular weight: 80.063 IUPAC Standard InChI: InChI=1S/O3S/c1-4 (2)3 IUPAC Standard InChIKey: AKEJUJNQAAGONA-UHFFFAOYSA-N CAS Registry Number: 7446-11-9 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .

So3 sulfur trioxide molecule Royalty Free Vector Image
SO3 +H2O → H2SO4 Due to the vigorous fuming of gaseous sulfur trioxide, the freshly formed sulfuric acid is in the form of mist. 2. Hydrofluorination: This is the same as hydration, but instead of water HF is added to SO 3 which forms fluorosulfuric acid. SO3 + HF → FSO3H 3.

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
Sulfur trioxide. Molecular Formula O. 3. S. Average mass 80.063 Da. Monoisotopic mass 79.956818 Da. ChemSpider ID 23080.

What Type Of Reaction Happens When Sulfur Dioxide Gas Reacts With
Reaction Information Word Equation Sulfur Dioxide + Dioxygen = Sulfur Trioxide SO2 + O2 = SO3 is a Synthesis reaction where two moles of Sulfur Dioxide [SO 2] and one mole of Dioxygen [O 2] combine to form two moles of Sulfur Trioxide [SO 3] Show Chemical Structure Image Reaction Type Synthesis Redox Reversible reaction (equilibrium)
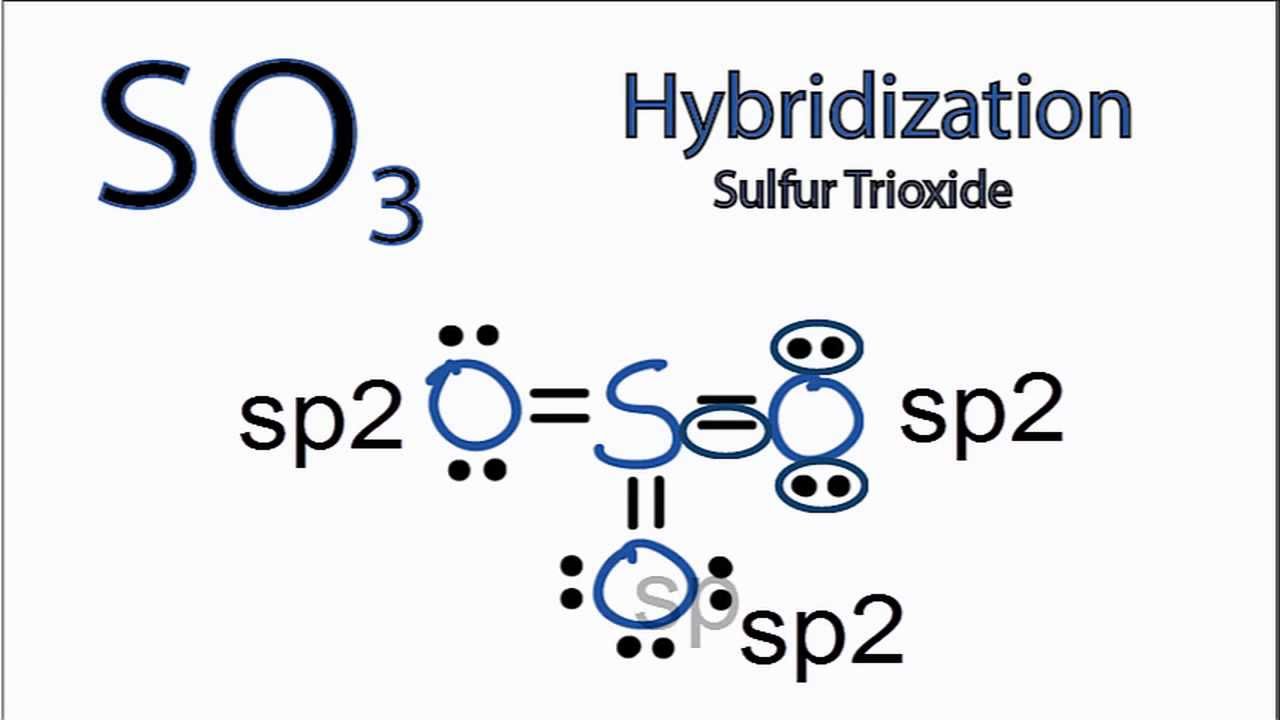
SO3 Hybridization Hybrid Orbitals for SO3 (sulfur trioxide) YouTube
Henry Agnew (UC Davis) 3.9: Chemical Equations is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. A chemical reaction is the process in which one or more substances are changed into one or more new substances. Chemical reactions are represented by chemical equations. Chemical equations have..
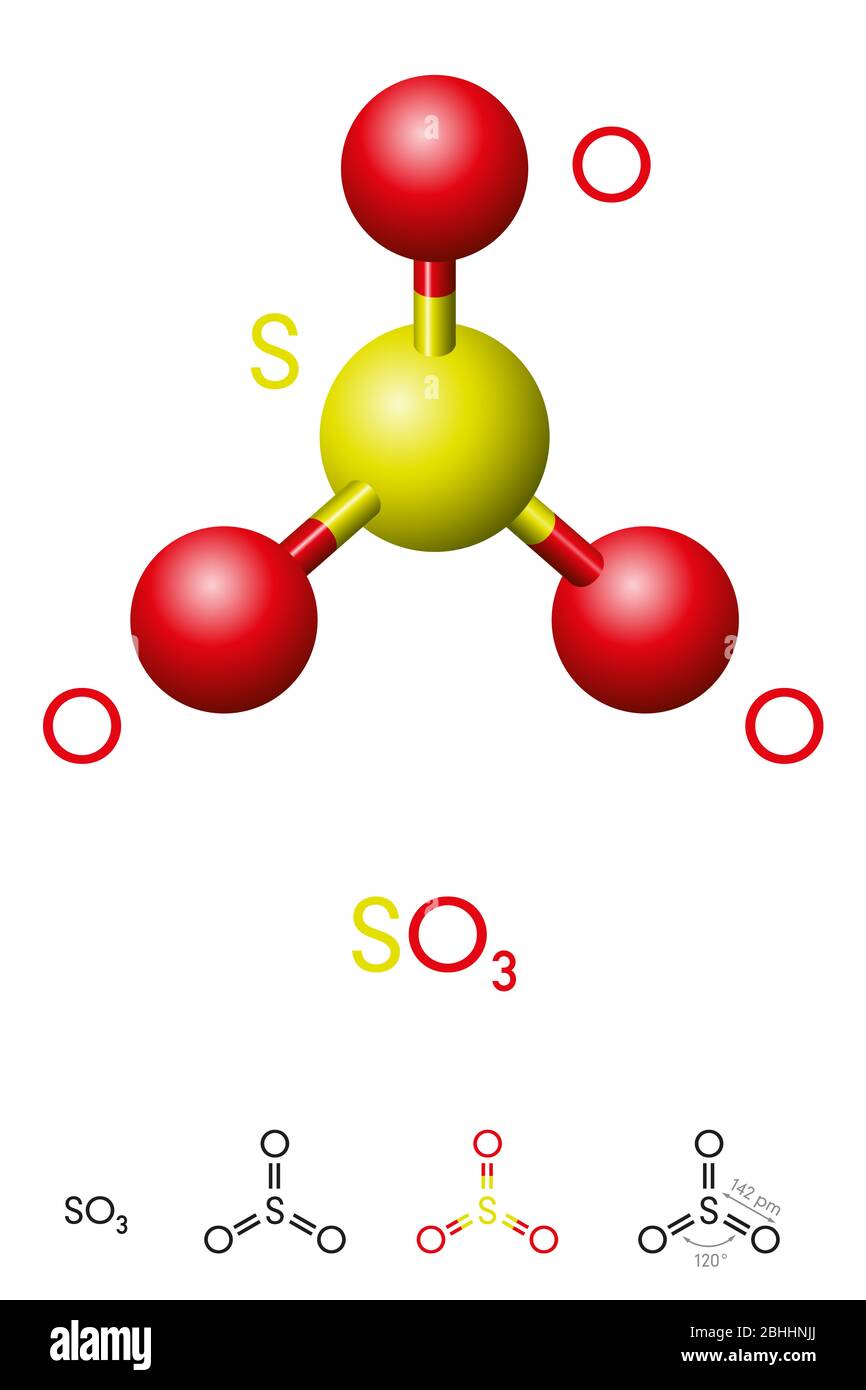
Sulfur trioxide, SO3, molecule model and chemical formula. Significant
129 Share 23K views 5 years ago In this video we'll write the correct formula for (Sulfur Trioxide) SO3. To write the formula for Sulfur Trioxidewe'll use the Periodic Table and follow some.

So3 sulfur trioxide molecule Royalty Free Vector Image
Instructor Laura Foist View bio Learn what sulfur trioxide is and understand the formula for sulfur trioxide. Find common sulfur trioxide reactions and see sulfur trioxide preparation.

S +O2 =SO3 Balanced EquationSulphur+Oxygen=Sulphur trioxide Balanced
Sulfur Trioxide + Water = Sulfuric Acid SO3 + H2O = H2SO4 is a Synthesis reaction where one mole of Sulfur Trioxide [SO 3] and one mole of Water [H 2 O] combine to form one mole of Sulfuric Acid [H 2 SO 4] Show Chemical Structure Image Reaction Type Synthesis Reactants Sulfur Trioxide - SO 3